-
工业上以黄铜矿(主要成分是CuFeS2,杂质不溶于水和酸)为原料,制备蓝色晶体G,其化学式为[Cu(NH3)4]SO4·H2O,涉及流程如下:
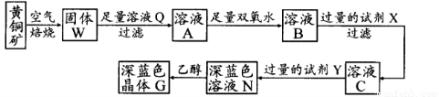
已知25℃时,几种金属氢氧化物的溶度积常数和完全沉淀的pH范围如下表:
(1)黄铜矿在空气中焙烧能生成铁和铜的低价硫化物,写出其反应的化学方程式________________________________________________________________________________________________________________________________________________________;
(2)试剂X的化学式为 ________________________________________________________________________________________________________________,双氧水的作用是________________________________________________________________________________________ ;
(3)常温下,0.1 mol/L试剂Y的pH=11,则该温度下,试剂Y的电离常数为________________________________________________________________________________________________________________________________,用pH试纸测该溶液pH值的方法是________________________________________________________________________________________________________________________________________________________________________________________________________________________;
(4)在溶液N中加入乙醇的目的是________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________。
-
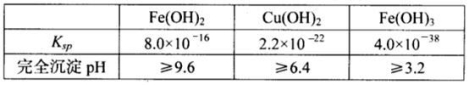
工业上以黄铜矿(主要成分是CuFeS2,杂质不溶于水和酸)为原料,制备蓝色晶体G,其化学式为[Cu(NH3)4]SO4·H2O,涉及流程如下:
已知25 ℃时,几种金属氢氧化物的溶度积常数和完全沉淀的pH范围如下表:
| Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
| Ksp | 8.0×10-16 | 2.2×10-20 | 4.0×10-38 |
| 完全沉淀pH | ≥9.6 | ≥6.4 | ≥3.2 |
(1)加快黄铜矿焙烧速率,可采用的措施有 (写两种)。
(2)加入双氧水可能发生反应的离子方程式为 ;
试剂X的化学式为 。
(3)常温下,0.1 mol/L试剂Y的pH=11,则该温度下,试剂Y的电离常数为 ,用pH试纸测该溶液pH的方法是 。
(4)已知Cu(OH)2+4NH3·H2O [Cu(NH3)4]2++2OH-+4H2O,写出该反应的平衡常数表达式: 。
[Cu(NH3)4]2++2OH-+4H2O,写出该反应的平衡常数表达式: 。
(5)在溶液N中加入乙醇的目的是 。
-
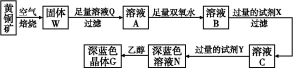
工业上以黄铜矿(主要成分是CuFeS2,杂质不溶于水和酸)为原料,制备蓝色晶体G,其化学式为[Cu(NH3)4]SO4·H2O,涉及流程如下:
已知25℃时,几种金属氢氧化物的溶度积常数和完全沉淀的pH范围如下表:
| Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
| Ksp | 8.0×10-16 | 2.2×10-22 | 4.0×10-38 |
| 完全沉淀pH | ≥9.6 | ≥6.4 | ≥3.2 |
(1)加快黄铜矿焙烧速率,可采用的措施有________(写两种):
(2)加入双氧水可能发生反应的离子方程式为________;
试剂X的化学式为________。
(3)常温下,0.1 mol/L试剂Y的pH=11,则该温度下,试剂Y的电离常数为________;
用pH试纸测该溶液pH值的方法是________
(4)已知Cu(OH)2+4NH3·H2O [Cu(NH3)4]2++2OH-+4H2O,写出该反应的平衡常数表达式:________。
[Cu(NH3)4]2++2OH-+4H2O,写出该反应的平衡常数表达式:________。
(5)在溶液N中加入乙醇的目的是________。
-
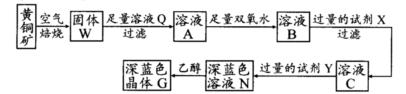
工业上以黄铜矿(主要成分是CuFeS2,杂质不溶于水和酸)为原料,制备蓝色晶体G,其化学式为[Cu(NH3)4]SO4·H2O,涉及流程如下:
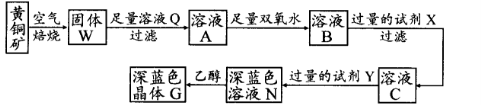
已知25℃时,几种金属氢氧化物的溶度积常数和完全沉淀的pH范围如下表:
(1)黄铜矿在空气中焙烧能生成铁和铜的低价硫化物,写出其反应的化学方程式: ;
(2)试剂X的化学式为 ;
(3)常温下,0.1 mol/L试剂Y的pH=11,则该温度下,试剂Y的电离常数为 ,用pH试纸测该溶液pH值的方法是 ;
(4)在溶液N中加入乙醇的目的是 。
-
铜及其化合物在工业上有许多用途。回答下列问题:
(1)某工厂以辉铜矿(主要成分为Cu2S,含少量Fe2O3、SiO2等杂质)为原料制备不溶于水的碱式碳酸铜的流程如下:
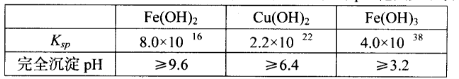
①浸取反应中氧化剂的化学式为 ;滤渣Ⅰ的成分为MnO2和 (写化学式)。
②“除铁”这一步反应在25℃进行,加入的试剂A为__________,若加A后溶液的PH调为4,溶液中Fe3+离子浓度为 mol/L。(已知Ksp[Fe(OH)3]= 4.0×10-38)
③“沉锰”(除Mn2+)过程中反应的离子方程式 。
④ 滤液Ⅱ经蒸发结晶得到的盐主要是 (写化学式)。
(2)某实验小组同学用电化学原理模拟湿法炼铜,进行了一系列探究活动。
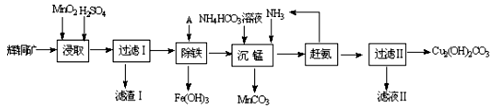
①下图1为某实验小组设计的原电池装置,盐桥内装载的是足量用饱和氯化钾溶液浸泡的琼脂,反应前电极质量相等,一段时间后,两电极质量相差25.8g,则导线中通过了_____ mol电子,若不考虑甲、乙两池电解质溶液中的离子向盐桥中移动,则乙池电解质溶液的质量与实验前的电解质溶液的质量差△m= g。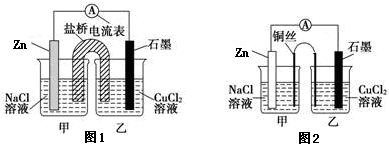
②其他条件不变,若将盐桥换成光亮的U形弯铜丝浸入甲池与乙池,如图2所示,电流计指针偏转方向与先前一样,但偏转角度明显减小。一段时间后,乙池石墨棒浸入液面以下部分也析出了一层紫红色固体,则甲池铜丝附近溶液的pH___(填“减小”、“增大”或“不变”) ,乙池中石墨为_____极(填“正”、“负”、“阴”或“阳”) 。
-
铜及其化合物在工业上有许多用途。回答下列问题:
(1)某工厂以辉铜矿(主要成分为Cu2S,含少量Fe2O3、SiO2等杂质)为原料制备不溶于水的碱式碳酸铜的流程如下:
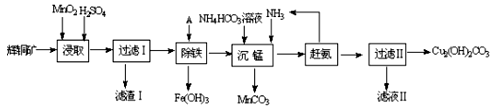
①浸取反应中氧化剂的化学式为 ;滤渣Ⅰ的成分为MnO2和 (写化学式)。
②“除铁”这一步反应在25℃进行,加入的试剂A为__________,若加A后溶液的PH调为4,溶液中Fe3+离子浓度为 mol/L。(已知Ksp[Fe(OH)3]= 4.0×10-38)
③“沉锰”(除Mn2+)过程中反应的离子方程式 。
④ 滤液Ⅱ经蒸发结晶得到的盐主要是 (写化学式)。
(2)某实验小组同学用电化学原理模拟湿法炼铜,进行了一系列探究活动。
①下图1为某实验小组设计的原电池装置,盐桥内装载的是足量用饱和氯化钾溶液浸泡的琼脂,反应前电极质量相等,一段时间后,两电极质量相差25.8g,则导线中通过了_____ mol电子,若不考虑甲、乙两池电解质溶液中的离子向盐桥中移动,则乙池电解质溶液的质量与实验前的电解质溶液的质量差△m= g。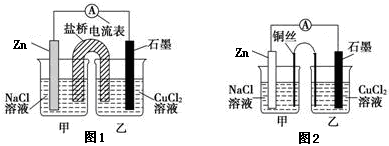
②其他条件不变,若将盐桥换成光亮的U形弯铜丝浸入甲池与乙池,如图2所示,电流计指针偏转方向与先前一样,但偏转角度明显减小。一段时间后,乙池石墨棒浸入液面以下部分也析出了一层紫红色固体,则甲池铜丝附近溶液的pH___(填“减小”、“增大”或“不变”) ,乙池中石墨为_____极(填“正”、“负”、“阴”或“阳”) 。
-
铜及其化合物在工业上有许多用途。某工厂以辉铜矿(主要成分为 Cu2S,含少量 Fe2O3、SiO2 等杂质)为原料制备不溶于水的碱式碳酸铜的流程如下:
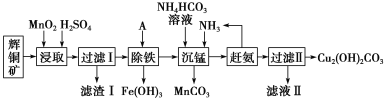
(1)“浸取”反应中氧化剂的化学式为________;若“浸取”反应中得到一种单质,则滤渣Ⅰ的成分为 MnO2 和________、________ (写化学式)。
(2)“除铁”这一步反应在 25 ℃ 进行,加入的试剂 A 为________,若加 A 后溶液 的 pH调为 4,溶液中 Fe3+浓度为______mol/L。{已知 Ksp[Fe(OH)3]=4.0×10-38}
(3)写出“沉锰”(除 Mn2+)过程中反应的离子方程式:________________________________。
(4)滤液Ⅱ经蒸发结晶得到的盐主要是__________(写化学式)。
(5)过滤Ⅱ的沉淀经过洗涤、干燥可以得到碱式碳酸铜,如何判断沉淀已洗净?_____________________________。
-
铜及其化合物在工业上有许多用途。回答下列问题:
(1)某工厂以辉铜矿(主要成分为Cu2S,含少量Fe2O3、SiO2等杂质)为原料制备不溶于水的碱式碳酸铜的流程如下:
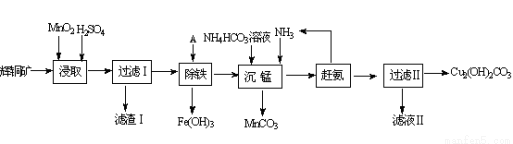
①浸取反应中氧化剂的化学式为________ ;滤渣Ⅰ的成分为MnO2、S和___________(写化学式)。
②“除铁”这一步反应在25℃进行,加入试剂A调节溶液PH为4后,溶液中铜离子最大浓度不超过_______ mol/L。(已知Ksp[Cu(OH)2]=2.2×10-20)
③“沉锰”(除Mn2+)过程中反应的离子方程式_________________________ 。
④滤液Ⅱ经蒸发结晶得到的盐主要是______________ (写化学式)。
(2)某实验小组同学用电化学原理模拟湿法炼铜,进行了一系列探究活动。
①如下左图为某实验小组设计的原电池装置,盐桥内装载的是足量用饱和氯化钾溶液浸泡的琼脂,反应前,电极质量相等,一段时间后,两电极质量相差6.00 g,则电解过程中盐桥中Cl-移向______________(填“甲池”或“乙池”)
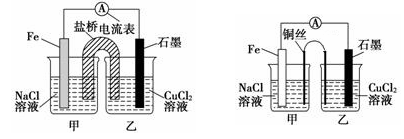
②其他条件不变,若将盐桥换成光亮的U形弯铜丝浸入甲池与乙池,如上右图所示,电流计指针偏转方向与先前一样,但偏转角度明显减小。一段时间后,乙池石墨棒浸入液面以下部分也析出了一层紫红色固体,则甲池铜丝附近溶液的pH________(填“减小”、“增大”或“不变”) ,乙池中石墨为________极(填“正”、“负”、“阴”或“阳”)。
-
(14分)工业上用辉铜矿(主要成分Cu2S,含Fe3O4、SiO2杂质)为原料,生产硫酸铜晶体的工艺流程如下:
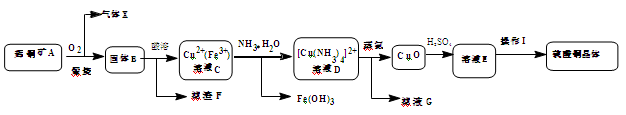
已知:①固体B为氧化物组成的混合物 ②[Cu(NH3)4]2+(aq)  Cu2+(aq) + 4NH3(aq)
Cu2+(aq) + 4NH3(aq)
(1)气体X是 ,高温下在过量空气中煅烧辉铜矿时,Cu2S发生反应的方程式为: 。
(2)固体B酸溶时加入稀硫酸和H2O2,目的是 ,不用浓硫酸的原因是 。
(3)鉴别溶液D中Fe3+完全除尽的方法是 。滤液G的主要溶质是 (填化学式)。
(4)由溶液E获得硫酸铜晶体的实验操作I的方法是蒸发浓缩、降温结晶、 、烘干。
(5)用“间接碘量法”测定所制备的CuSO4·5H2O(不含能与I-反应的氧化性杂质)的纯度。取a g试样配成100 mL溶液,取25.00 mL该溶液,滴加KI溶液后有白色碘化物沉淀生成,滴加KI溶液至沉淀不再产生为止,然后用硫代硫酸钠标准溶液滴定生成的I2,发生反应的化学方程式为I2+2Na2S2O3===2NaI+Na2S4O6,消耗c mol·L-1 Na2S2O3溶液的体积为V mL。
①写出CuSO4与KI反应的离子方程式_________________________。
②计算试样中CuSO4·5H2O的纯度____________________(用a、c、V表示)。
-
铜及其化合物在工业生产上有许多用途。某工厂以辉铜矿(主要成分为Cu2S,含少量Fe2O3、SiO2等杂质)为原料制备不溶于水的碱式碳酸铜的流程如图:
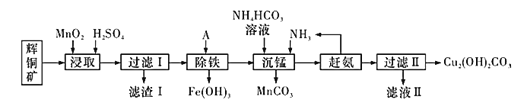
(1)加快“浸取”速率除将辉铜矿粉碎外,还可采取的措施有_________ (任写一种);研究发现若先除铁再浸取,浸取速率明显变慢,其可能原因是________。
(2)滤渣I中的主要成分是MnO2、S、SiO2。请写出“浸取”反应中生成S的化学方程式:________。
(3)常温下“除铁”时加入的试剂A可用____________较为合适,若加A后溶液的pH调为5,则溶液中Fe3+ 的浓度为______mol/L。{Ksp[Fe(OH)3]=4.0×10- 38}
(4)写出“沉锰"(除Mn2+ )过程中反应的离子方程式:______。
(5)“赶氨”时,最适宜的操作方法是__________。
(6)滤液II经蒸发结晶得到的盐主要是________(写化学式)。














