
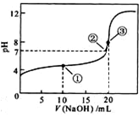
A.点①所示溶液:c(CH3COO-)+c(CH3COOH)=c(Na+)
B.点②所示溶液:c(Na+)=c(CH3COO-)>c(H+)=c(OH-)
C.点③所示溶液:c(Na+)>c(OH-)>c(CH3COO-)>c(H+)
D.滴定终点时:c(CH3COOH)+c(CH3COO-)=c(Na+)
高三化学选择题中等难度题

高三化学选择题中等难度题
常温下,用 0.1000 mol·L-1NaOH 溶液滴定 20.00 mL 0.1000 mol·L-1的 CH3COOH 溶液所得滴定曲线如图。下列说法正确的是
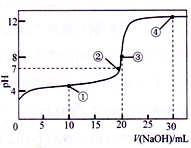
A. 点①所示的溶液中:c(Na+)+c(H+)>c(CH3COOH)+c(OH-)
B. 点②所示溶液中:c(Na+)=c(CH3COO-)+c(CH3COOH)
C. 点③所示溶液中:c(H+)=c(CH3COOH)+c(OH-)
D. 点④所示溶液中:2c(OH-)-2c(H+)=c(CH3COO-)+3c(CH3COOH)
高三化学选择题困难题查看答案及解析
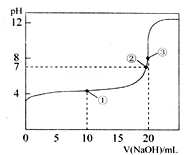
常温下,用0.1000 mol·L-1 NaOH溶液滴定20.00 mL 0.1000 mol·L-1 CH3COOH溶液所得滴定曲线如图。下列说法不正确的是( )
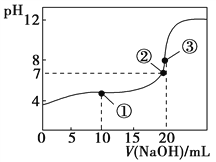
A. 在曲线上任一点均存在:c(Na+)−c(OH-) = c(CH3COO-)−c(H+)
B. 点①所示溶液中:c(CH3COO-)+2c(OH-) = c(CH3COOH)+2c(H+)
C. 点②所示溶液中:c(Na+) = c(CH3COO-)
D. 点③所示溶液中:c(Na+) > c(OH-) > c(CH3COO-) > c(H+)
高三化学单选题中等难度题查看答案及解析
常温下,用0.1000 mol·L-1 NaOH溶液滴定20.00 mL 0.1000 mol·L-1 CH3COOH溶液所得滴定曲线如图。下列说法不正确的是( )
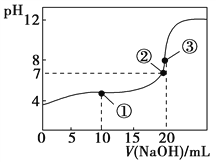
A.在曲线上任一点均存在:c(Na+)−c(OH-) = c(CH3COO-)−c(H+)
B.点①所示溶液中:c(CH3COO-)+2c(OH-) = c(CH3COOH)+2c(H+)
C.点②所示溶液中:c(Na+) = c(CH3COO-)
D.点③所示溶液中:c(Na+) > c(OH-) > c(CH3COO-) > c(H+)
高三化学单选题中等难度题查看答案及解析
常温下,用0.1000 mol·L-1 NaOH溶液滴定20.00 mL 0.1000 mol·L-1 CH3COOH溶液所得滴定曲线如图。下列说法不正确的是( )
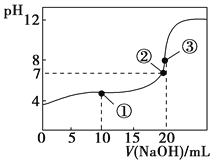
A.在曲线上任一点均存在:c(Na+)−c(OH-) = c(CH3COO-)−c(H+)
B.点①所示溶液中:c(CH3COO-)+2c(OH-) = c(CH3COOH)+2c(H+)
C.点②所示溶液中:c(Na+) = c(CH3COO-)
D.点③所示溶液中:c(Na+) > c(OH-) > c(CH3COO-) > c(H+)
高三化学单选题中等难度题查看答案及解析
常温下,用0.1000 mol·L-1 NaOH溶液滴定20.00 mL 0.1000 mol·L-1 CH3COOH溶液所得滴定曲线如图。下列说法不正确的是( )
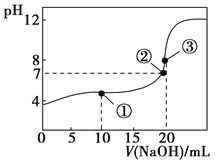
A. 在曲线上任一点均存在:c(Na+)−c(OH-) = c(CH3COO-)−c(H+)
B. 点①所示溶液中:c(CH3COO-)+2c(OH-) = c(CH3COOH)+2c(H+)
C. 点②所示溶液中:c(Na+) = c(CH3COO-)
D. 点③所示溶液中:c(Na+) > c(OH-) > c(CH3COO-) > c(H+)
高三化学单选题中等难度题查看答案及解析
高三化学选择题中等难度题查看答案及解析

高三化学选择题中等难度题查看答案及解析
常温下,用0.1000mol·L-1NaOH溶液滴定20.00mL0.1000 mol·L-1的CH3COOH溶液所得滴定曲线如图。下列说法正确的是
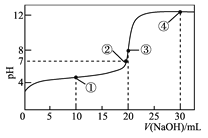
A. 点①所示溶液中:c(Na+)+c(H+)<CH3COOH)+c(OH-)
B. 点②所示溶液中:c(Na+)=c(CH3COO-)+c(CH3COOH)
C. 点③所示溶液中:c(H+)= c(CH3COOH)+c(OH-)
D. 点④所示溶液中:2c(OH-)-2c(H+)= c(CH3COO-)+3c(CH3COOH)
高三化学单选题困难题查看答案及解析

高三化学选择题中等难度题查看答案及解析
高三化学选择题中等难度题查看答案及解析