N2和H2合成NH3的能量变化如图所示,该反应的热化学方程式是( )

A. N2(g)+3H2(g) = 2NH3(g) ;△H = 2(b—a) kJ/mol
B. N2(g)+3H2(g) = 2NH3(l);△H = 2(a—b—c) kJ/mol
C. N2(g)+
H2(g) = NH3(l) ;△H = (b+c—a) kJ/mol
D. N2(g)+
H2(g) =NH3(g) ;△H = (a+b) kJ/mol
高二化学单选题简单题
N2和H2合成NH3的能量变化如图所示,该反应的热化学方程式是( )

A. N2(g)+3H2(g) = 2NH3(g) ;△H = 2(b—a) kJ/mol
B. N2(g)+3H2(g) = 2NH3(l);△H = 2(a—b—c) kJ/mol
C. N2(g)+
H2(g) = NH3(l) ;△H = (b+c—a) kJ/mol
D. N2(g)+
H2(g) =NH3(g) ;△H = (a+b) kJ/mol
高二化学单选题简单题
合成氨反应过程中的能量变化如图所示,下列说法正确的是

A. 反应体系中加入催化剂,会改变反应的热效应
B. 反应物的总能量低于生成物的总能量
C. 该反应的热化学方程式为3H2(g)+N2(g)2NH3(g)△H=-QkJ/mol(Q>0)
D. 该反应是吸热反应
高二化学单选题中等难度题查看答案及解析
已知合成氨的反应为:N2 + 3H2 2NH3 ΔH= - 92.4 KJ/mol在一定条件下达到化学平衡,现升高温度使平衡发生移动,下列图像中能正确描述正、逆反应速率(v)变化的是
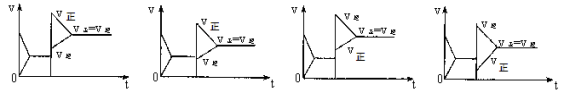
A B C D
高二化学选择题中等难度题查看答案及解析
N2和H2合成NH3的能量变化如图所示,该反应的热化学方程式是( )
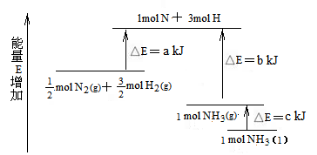
A.N2(g)+3H2(g) = 2NH3(g) ;△H = 2(b—a) kJ/mol
B.N2(g)+3H2(g) = 2NH3(l);△H = 2(a—b—c) kJ/mol
C.N2(g)+
H2(g) = NH3(l) ;△H = (b+c—a) kJ/mol
D.N2(g)+
H2(g) =NH3(g) ;△H = (a+b) kJ/mol
高二化学选择题困难题查看答案及解析
合成氨工业对国民经济和社会发展具有重要的意义。对于密闭容器中的反应:N2(g) + 3H2(g) 2NH3(g),500K、30MPa下n(NH3)、n(H2)和n(N2)随时间变化的关系如图所示。请回答下列问题:
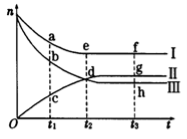
(1)上图中属于氢气物质的量随时间变化的曲线是 (填“I”、“II”或“Ⅲ”)。
(2)关于t2时刻的说法正确的是
A.t2时该反应达到平衡状态
b.t2时II和III代表的物质反应速率相同
c.t2时氢气、氮气与氨气的反应速率之比为3:1:2
D.t2时氨气与氢气的物质的量相同
(3)对于II所代表的物质,其逆反应速率最大的点是__________(填“c”、“d”或“g”);对于I所代表的物质,其正反应速率最小的点是___________ (填“a”、“e”或“f”)。
(4)其他条件不变,只改变温度,在改变的这个温度下反应至t3时刻,此时n(H2)比图象中的值大,那么该温度可能是____________(填序号)
A.673 K B.273 K C.373 K
(5)在密闭容器中充入2 mol N2和6 mol H2,—定条件下建立平衡:N2(g) + 3H2(g) 2NH3(g) △H=-92.2kJ/mol,测得 N2 的转化率为 90%,则在此条件下,反应放出的热量为___________kJ。若与上述反应的温度和体积相同时,向密闭容器中充入4 mol NH3,则达平衡后,反应_________(填“放出”或“吸收”)的热量为___________kJ。
高二化学填空题困难题查看答案及解析
N2和H2合成NH3的能量变化如图所示,该反应的热化学方程式是( )

A.N2(g)+3H2(g)=2NH3(l) △H=2(a-b-c)kJ/mol
B.N2(g)+3H2(g)=2NH3(g) △H=2(b-a)kJ/mol
C.1/2 N2(g)+3/2H2(g)=NH3(l) △H=(b+c-a)kJ/mol
D.1/2 N2(g)+3/2H2(g)=NH3(g) △H=(a+b)kJ/mol
高二化学单选题中等难度题查看答案及解析
N2和H2合成NH3的能量变化如图所示,该反应的热化学方程式是
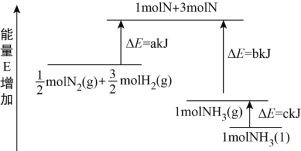
A. N2(g)+3H2(g)2NH3(l) ΔH=2(a-b-c) kJ·mol-1
B. N2(g)+3H2(g)2NH3(g) ΔH=2(b-a) kJ·mol-1
C. 1/2N2(g)+3/2H2(g)NH3(l) ΔH=(b+c-a) kJ·mol-1
D. 1/2N2(g)+3/2H2(g)NH3(g) ΔH=(a+b) kJ·mol-1
高二化学单选题中等难度题查看答案及解析
N2和H2合成NH3的能量变化如图所示,该反应的热化学方程式是( )

A. N2(g)+3H2(g) = 2NH3(g) ;△H = 2(b—a) kJ/mol
B. N2(g)+3H2(g) = 2NH3(l);△H = 2(a—b—c) kJ/mol
C. N2(g)+
H2(g) = NH3(l) ;△H = (b+c—a) kJ/mol
D. N2(g)+
H2(g) =NH3(g) ;△H = (a+b) kJ/mol
高二化学单选题简单题查看答案及解析
已知:3H2(g)+N2(g)⇌2NH3(g)△H=-92kJ/mol,在催化剂存在时反应过程中的能量变化如图所示。下列叙述正确的是
A.△H=E2-E1+E3-E4
B.加入催化剂后反应经过两步完成,其中第一步反应决定总反应速率
C.加入催化剂,△H、反应速率均发生改变
D.向密闭容器中充入3 mol H2和1molN2,发生上述反应,达到平衡时,反应放出92 kJ热量
高二化学单选题中等难度题查看答案及解析
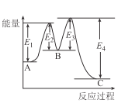
已知工业合成氨的方程式:3H2(g)+N2(g) 2NH3(g),该反应在一定条件下进行时的热效应如图1,所示下列说法正确的是

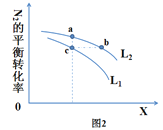
A. 由图1可知,该逆反应的活化能为(E+ΔH) kJ/mol
B. 图2中L、X表示的物理量是温度或压强,依据信息可判断L1>L2
C. 图2中a、b、c三点中υ正最大的点是b
D. 图2中a、b、c点平衡常数的关系为:Ka=Kc<Kb
高二化学选择题困难题查看答案及解析
合成氨反应:N2(g) + 3H2(g) 2NH3(g) △H=-92.4kJ·mol-1,在反应过程中,正反应速率的变化如下图:
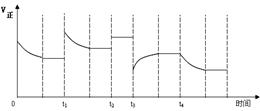
下列说法正确的是
A.t1时增大了压强
B.t2时同倍数增加N2、H2的物质的量
C.t3时降低了温度
D.t4时增大了c(NH3)
高二化学选择题简单题查看答案及解析