已知:Fe(s)+O2(g)═FeO(s)△H1=-272kJ/mol,2Al(s)+
O2(g)═Al2O3(s)△H2=-1 675kJ/mol,则2Al(s)+3FeO(s)═Al2O3(s)+3Fe(s)的△H是( )
A.-859 kJ/mol B.859 kJ/mol
C.-1403 kJ/mol D.-2491 kJ/mol
高二化学选择题中等难度题
已知:Fe(s)+O2(g)═FeO(s)△H1=-272kJ/mol,2Al(s)+
O2(g)═Al2O3(s)△H2=-1 675kJ/mol,则2Al(s)+3FeO(s)═Al2O3(s)+3Fe(s)的△H是( )
A.-859 kJ/mol B.859 kJ/mol
C.-1403 kJ/mol D.-2491 kJ/mol
高二化学选择题中等难度题
已知:Fe(s)+O2(g)═FeO(s)△H1=-272kJ/mol,2Al(s)+
O2(g)═Al2O3(s)△H2=-1 675kJ/mol,则2Al(s)+3FeO(s)═Al2O3(s)+3Fe(s)的△H是( )
A.-859 kJ/mol B.859 kJ/mol
C.-1403 kJ/mol D.-2491 kJ/mol
高二化学选择题中等难度题查看答案及解析
已知:Fe(s)+O2(g) = FeO(s) ΔH1=-272kJ/mol
2Al(s)+O2(g)= Al2O3(s) ΔH2=-1675kJ/mol
则2Al(s) +3FeO(s) = Al2O3(s)+ 3Fe(s)的ΔH是
A. -859kJ/mol B. 859kJ/mol C. -1403kJ/mol D. -2491kJ/mol
高二化学选择题简单题查看答案及解析
已知下列数据:
Fe(s)+O2(g) =FeO(s) △H=-272kJ·mol-1
2Al (s)+O2(g) =Al2O3(s) △H=-1675kJ·mol-1
则2Al(s) +3FeO(s) =Al2O3(s) + 3Fe(s)的△H是( )
A.+859 kJ·mol-1 B.-859 kJ·mol-1
C.-1403 kJ·mol-1 D.-2491 kJ·mol-1
高二化学选择题简单题查看答案及解析
已知下列数据:
Fe(s)+O2(g)=FeO(s) △H=-272kJ·mol-1
2Al(s)+O2(g)=Al2O3(s) △H=-1675kJ·mol-1
则2Al(s) +3FeO(s)=Al2O3(s) + 3Fe(s)的△H是
A.+859 kJ·mol-1 B.-859 kJ·mol-1
C.-1403 kJ·mol-1 D.-2491 kJ·mol-1
高二化学选择题中等难度题查看答案及解析
已知:下列两个热化学方程式:
Fe(s)+O2(g)═FeO(s) △H=﹣272.0KJ/mol
2Al(s)+O2(g)═Al2O3(s) △H=﹣1675.7KJ/mol
则 Al(s)的单质和FeO(s)反应的热化学方程式是 .
高二化学填空题简单题查看答案及解析
已知:Fe(s)+O2(g)===FeO(s) ΔH1=-272 kJ/mol
2Al(s)+O2(g)===Al2O3(s) ΔH2=-1 675 kJ/mol
则2Al(s)+3FeO(s)===Al2O3(s)+3Fe(s)的ΔH是
A.859 kJ/mol B.-859 kJ/mol
C.-1403 kJ/mol D.-2491 kJ/mol
高二化学选择题中等难度题查看答案及解析
(11分)(1)已知:①Fe(s)+O2(g)=FeO(s) ΔH1=-272.0 kJ·mol-1;
②2Al(s)+O2(g)===Al2O3(s) ΔH2=-1675.7 kJ·mol-1。
Al和FeO发生铝热反应的热化学方程式是 。
某同学认为,铝热反应可用于工业炼铁,你的判断是 (填“能”或“不能”),
你的理由是 。
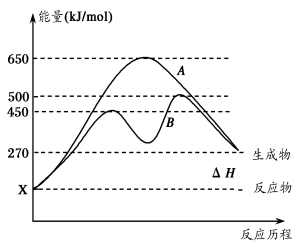
(2)反应物与生成物均为气态的某可逆反应在不同条件下的反应历程分别为A、B,
如图所示。

①据图判断该反应是___(填“吸”或“放”)热反应,当反应达到平衡后,其他
条件不变,升高温度,逆反应速率将____(填“增大”、“减小”或“不变”)。
②其中B历程表明此反应采用的条件为________(填字母)。
A.升高温度 B.增大反应物的浓度
C.降低温度 D.使用催化剂
(3)已知热化学方程式:H2(g)+O2(g)=H2O(g) ΔH=-241.8 kJ·mol-1
该反应的活化能为167.2 kJ·mol-1,则其逆反应的活化能为 。
高二化学填空题中等难度题查看答案及解析
(11分)(1)已知:①Fe(s)+O2(g)=FeO(s) ΔH1=-272.0 kJ·mol-1;
②2Al(s)+O2(g)===Al2O3(s) ΔH2=-1675.7 kJ·mol-1。
Al和FeO发生铝热反应的热化学方程式是 。
某同学认为,铝热反应可用于工业炼铁,你的判断是 (填“能”或“不能”),
你的理由是 。
(2)反应物与生成物均为气态的某可逆反应在不同条件下的反应历程分别为A、B,
如图所示。
①据图判断该反应是___(填“吸”或“放”)热反应,当反应达到平衡后,其他
条件不变,升高温度,逆反应速率将____(填“增大”、“减小”或“不变”)。
②其中B历程表明此反应采用的条件为________(填字母)。
A.升高温度 B.增大反应物的浓度
C.降低温度 D.使用催化剂
(3)已知热化学方程式:H2(g)+O2(g)=H2O(g) ΔH=-241.8 kJ·mol-1
该反应的活化能为167.2 kJ·mol-1,则其逆反应的活化能为 。
高二化学填空题中等难度题查看答案及解析
已知下列数据:Fe(s) + 1/2O2(g) = FeO(s) ΔH=-272kJ/mol;2Al(s) + 3/2O2(g) = Al2O3(s) ΔH=-1675kJ/mol;则2Al(s) + 3FeO(s) = Al2O3(s) + 3Fe(s)的ΔH是
A.-859 kJ/mol B.+859 kJ/mol C.-1403 kJ/mol D.-2491 kJ/mol
高二化学选择题中等难度题查看答案及解析
(2分)已知:下列两个热化学方程式:
Fe(s) + 1/2O2(g) FeO(s)
=-272.0KJ/mol
2Al(s) + 3/2O2(g) Al2O3(s)
=-1675.7KJ/mol
则 Al(s)的单质和FeO(s)反应的热化学方程式是______________________________________________________ 。
高二化学填空题中等难度题查看答案及解析